Abstracts
Seasonal fluctuations in the dynamics of the plankton rotifer populations in an oligo-mesotrophic branch of a tropical reservoir were analyzed with respect to the possible influence of environmental conditions (physical, chemical and biological), with emphasis on biological interactions. Samples were taken monthly from August 2006 to July 2007. The well-defined climatic seasons were reflected in variations of the physical and chemical properties of the water. The zooplankton community consisted mainly of Rotifera, Cladocera and Copepoda, with occasional representatives of Chaoborus brasiliensis (Diptera), Ostracoda, Protozoa and Turbellaria. Rotifera was the dominant group in zooplankton community in 5 of the 12 months of the study and were represented by 35 taxa. Redundancy analysis between the rotifer population abundances and the set of environmental variables showed significant (p < 0.05) positive correlations with the chlorophyll a in the rainy season. In the dry period, predation was probably the environmental factor that had the strongest effect on rotifer populations; in particular, the density of the cyclopoid Thermocyclops decipiens showed significant (p < 0.05) negative correlations with many of the rotifer population abundances. In addition, analysis of the co-occurrence of different rotifer species indicated strong competitive interactions between populations, during the rainy period (p < 0.001). Among the biotic environmental factors analyzed, algal biomass had an important role during the rainy period and inter-rotifer competition was also significant in these months. It was probably that predation by the cyclopoid copepod Thermocyclops decipiens determined the structure and dynamics of the rotifer populations in the Sapucaí arm of the Furnas reservoir, during the dry period.
Rotifera; Furnas reservoir; intra-zooplankton predation; inter-specific competition
Mudanças na dinâmica sazonal dos rotíferos planctônicos em uma porção oligo-mesotrófica de um reservatório tropical foram avaliadas em relação às variáveis ambientais (físicas, químicas e biológicas). As coletas foram realizadas mensalmente de agosto de 2006 a julho de 2007. As estações climáticas foram bem definidas, refletindo em variações nas características físicas e químicas da água. A comunidade zooplanctônica foi representada pelos grupos: Rotifera, Cladocera, Copepoda, e ocasionalmente por Chaoborus brasiliensis (Diptera), Ostracoda, Protozoa e Turbellaria. Os Rotifera foram dominantes na comunidade em cinco dos 12 meses amostrados, tendo sido representados por 35 taxa. A análise de redundância entre as densidades das populações de Rotifera e as diversas variáveis ambientais indicou correlação positiva significativa (p < 0.05) com a concentração de clorofila a no período chuvoso. No período seco, provavelmente a predação foi o fator ambiental que mais influenciou estas populações, tendo sido obtida correlação negativa significativa (p < 0.05) entre a densidade da espécie Thermocyclops decipiens e a maior parte das populações de rotíferos. A análise de co-ocorrência entre as populações de rotíferos indicou fortes interações competitivas entre estas populações no período chuvoso (p < 0.001). Entre os fatores bióticos analisados a biomassa algal teve um papel importante durante o período chuvoso e a competição inter-específica entre os Rotifera também foi significativa neste período. No entanto, a predação pelo Copepoda Cyclopoida Thermocyclops decipiens foi provavelmente o que determinou a estrutura e dinâmica das populações de rotíferos no compartimento Sapucaí do reservatório de Furnas, durante o período seco.
Rotifera; reservatório de Furnas; predação intra-zooplanctônica; competição interespecífica
ARTIGOS
The influence of environmental factors on the seasonal dynamics and composition of Rotifera in the Sapucaí River arm of Furnas Reservoir, MG, Brazil
Influência de fatores ambientais na composição e dinâmica sazonal de Rotifera no compartimento Rio Sapucaí, UHE de Furnas, MG, Brasil
Natalia Felix NegreirosI, * * Corresponding author: Natalia Felix Negreiros, e-mail: natalia_felix@yahoo.com.br ; Maria José dos Santos-WisniewskiII; Renata Martins dos SantosI; Odete RochaIII
IPost-graduate Program in Ecology and Natural Resources, Federal University of São Carlos - UFSCar, São Carlos, SP, Brazil
IIDepartment of Biology, Federal University of Alfenas, Alfenas, MG, Brazil
IIIDepartment of Ecology and Evolutionary Biology, Federal University of São Carlos - UFSCar, São Carlos, SP, Brazil
ABSTRACT
Seasonal fluctuations in the dynamics of the plankton rotifer populations in an oligo-mesotrophic branch of a tropical reservoir were analyzed with respect to the possible influence of environmental conditions (physical, chemical and biological), with emphasis on biological interactions. Samples were taken monthly from August 2006 to July 2007. The well-defined climatic seasons were reflected in variations of the physical and chemical properties of the water. The zooplankton community consisted mainly of Rotifera, Cladocera and Copepoda, with occasional representatives of Chaoborus brasiliensis (Diptera), Ostracoda, Protozoa and Turbellaria. Rotifera was the dominant group in zooplankton community in 5 of the 12 months of the study and were represented by 35 taxa. Redundancy analysis between the rotifer population abundances and the set of environmental variables showed significant (p < 0.05) positive correlations with the chlorophyll a in the rainy season. In the dry period, predation was probably the environmental factor that had the strongest effect on rotifer populations; in particular, the density of the cyclopoid Thermocyclops decipiens showed significant (p < 0.05) negative correlations with many of the rotifer population abundances. In addition, analysis of the co-occurrence of different rotifer species indicated strong competitive interactions between populations, during the rainy period (p < 0.001). Among the biotic environmental factors analyzed, algal biomass had an important role during the rainy period and inter-rotifer competition was also significant in these months. It was probably that predation by the cyclopoid copepod Thermocyclops decipiens determined the structure and dynamics of the rotifer populations in the Sapucaí arm of the Furnas reservoir, during the dry period.
Keywords: Rotifera, Furnas reservoir, intra-zooplankton predation, inter-specific competition.
RESUMO
Mudanças na dinâmica sazonal dos rotíferos planctônicos em uma porção oligo-mesotrófica de um reservatório tropical foram avaliadas em relação às variáveis ambientais (físicas, químicas e biológicas). As coletas foram realizadas mensalmente de agosto de 2006 a julho de 2007. As estações climáticas foram bem definidas, refletindo em variações nas características físicas e químicas da água. A comunidade zooplanctônica foi representada pelos grupos: Rotifera, Cladocera, Copepoda, e ocasionalmente por Chaoborus brasiliensis (Diptera), Ostracoda, Protozoa e Turbellaria. Os Rotifera foram dominantes na comunidade em cinco dos 12 meses amostrados, tendo sido representados por 35 taxa. A análise de redundância entre as densidades das populações de Rotifera e as diversas variáveis ambientais indicou correlação positiva significativa (p < 0.05) com a concentração de clorofila a no período chuvoso. No período seco, provavelmente a predação foi o fator ambiental que mais influenciou estas populações, tendo sido obtida correlação negativa significativa (p < 0.05) entre a densidade da espécie Thermocyclops decipiens e a maior parte das populações de rotíferos. A análise de co-ocorrência entre as populações de rotíferos indicou fortes interações competitivas entre estas populações no período chuvoso (p < 0.001). Entre os fatores bióticos analisados a biomassa algal teve um papel importante durante o período chuvoso e a competição inter-específica entre os Rotifera também foi significativa neste período. No entanto, a predação pelo Copepoda Cyclopoida Thermocyclops decipiens foi provavelmente o que determinou a estrutura e dinâmica das populações de rotíferos no compartimento Sapucaí do reservatório de Furnas, durante o período seco.
Palavras-chave: Rotifera, reservatório de Furnas, predação intra-zooplanctônica, competição interespecífica.
Introduction
Rotifera constitutes an import part of the freshwater zooplankton and have an essential role in energy transfer and the regeneration and transport of nutrients into the food web, as they are largely composed of direct consumers of phytoplankton primary production. They are generally present in great numbers and with high species richness in tropical zooplankton, being opportunistic organisms capable of colonizing a wide variety of habitats, from temporarily flooded areas (Koste & Robertson 1990, Bonecker & Lansac-Tôha, 1996, Lima et al. 1996, Garcia et al. 1998, Martínez et al. 2000, Lansac-Tôha et al. 2009) to large rivers, lakes and reservoirs (Oliveira Neto & Moreno 1999, Aoyagui & Bonecker. 2004, Sendacz et al. 2006, Corgosinho & Pinto-Coelho, 2006, Almeida et al. 2009, Borges & Pedrozo, 2009).
The representatives of this group are small and short-lived (Streble & Krauter 1987) and generally show high sensitivity, responding quickly to various types of environmental change, thus altering the total density of zooplankton and modifying the species composition and diversity of the community (Coelho-Botelho 2004). Although the freshwater zooplankton community consists mainly of rotifers, cladocerans and copepods, other groups are present at lower densities and frequencies, such as turbellarians, water acarines and dipteran larvae (Chaoboridae, Culicidae) (Tundisi et al. 1988), and biotic interactions among them can be a relevant factors.
The composition and dynamics of a community are influenced by surrounding conditions and a complex of physical, chemical and biological factors, as well as interactions among them, that can play a major part in selecting the prevailing species (Lehman 1991). While organisms numbers and relative abundance are the fundamental properties that define the structure of a community, local processes of competition, predation and heterogeneity (in time and space) of the environment are the immediate determining factors of population dynamics in a given locality (Chesson 1986). Although in some particular environments, the physical and chemical variables may be more important than biotic variables, or vice versa, both sets of factors usually operate simultaneously along a seasonal cycle.
In the present study, the most important variables controlling the abundance of rotifers in the plankton communities, according to the literature were measured during a one-year period of in the Sapucaí arm of the Furnas hydroelectric power station reservoir, and analysed regarding its effects on the structure and dynamics of the Rotifera assemblages. The main focus was upon the variables indicating biotic interactions, such as herbivory (phytoplankton biomass), competition (other filter-feeders) and intrazooplankton predation (zooplankton carnivores), that could influence the dynamics of rotifer populations.
Material and Methods
1. Study area
The reservoir serving the Furnas hydroelectric power station, situated in the Rio Grande basin in Minas Gerais State, Brazil (20º 40' S and 46º 19' W) is formed by the Grande (northern arm) and Sapucaí (southern arm) rivers. A limnetic section of this later arm (Figure 1) was sampled monthly from August 2006 to July 2007.
2. Experimental design
The following physical and chemical properties of the water were measured in situ at each collection: temperature with a mercury thermometer; electrical conductivity and pH with Quimis digital meters. Samples of water were also collected for the measurement of dissolved oxygen (DO), total N, total P and chlorophyll a concentrations. DO concentration was determined by the Winkler method, modified with NaN3, as described by Golterman et al. (1978), and the phosphorus and nitrogen contents by the methods given in Mackereth et al. (1978). Chlorophyll a was determined by 90% acetone extraction (Lorenzen 1967, Golterman et al. 1978). Water transparency was measured using a Secchi disk of 30 cm of diameter.
The Trophic State Index (TSI) of Carlson (1977), modified by Toledo et al. (1983) was calculated using water transparency, chlorophyll a and total phosphorus concentrations.
Zooplankton was collected by vertical hauls with a 68 μm mesh plankton net through the whole water column. The volume of water filtered was estimated by multiplying the area of the net mouth by the local depth of the water. The organisms were fixed in 4% formaldehyde solution and later identified and counted under an optical Leica (DMLS) microscope (up to 1000X magnification), and specialized literature (Edmondson 1959, Koste 1978, Segers 1995, Elmoor-Loureiro 1997, Smirnov 1996, Rocha & Matsumura-Tundisi 1976, Matsumura-Tundisi 1986, Reid 1985). Cladocera and Copepoda were counted in square plastic counting dishes marked with a square 5 mm grid on the bottom, using sample aliquots of 5 mL or more and, in the case of the rare taxa, the whole sample. Rotifers were counted in a Sedgewick-Rafter cell, using sample aliquots of 1 mL and, at times, the whole sample.
3. Analysis of data
The statistical analysis was based on data from monthly samples, 19 species of rotifer and 20 environmental variables (5 physical and chemical factors, chlorophyll a content, 7 potential competitors and 7 potential predators). The data were subjected to redundancy analysis (RDA), employing the program CANOCO 3.12 (Ter Braak & milauer 2002). Rotifer species densities were transformed by the function log (x+1) before analysis. The Monte Carlo test, with 999 random permutations, was used to test the significance of the environmental variables.
In view of the great number of rotifer species found in the samples, Principal Components Analysis (PCA) was applied to rotifer densities, in order to select those species that accounted for more than 0.8 of variance for the first two components. To avoid overvaluing some RDA results with very different number of variables in different tests, the competitors (7 variables) were grouped into taxonomic families.
The variables total P and total N were treated as unreal independent environmental variables, as their values and those of total zooplankton density are mutually dependent. Thus, these variables were excluded from the RDA calculations. The program EcoSim® (Gotelli & Entsminger 2001) was used to verify the existence of competition among rotifer species during the period of study.
Results
During the study, the climatic periods were well defined, with the rainy season extending from October 2006 to February 2007. The dry period (August and September 2006 and March to July 2007) was marked by low temperatures and low pluviosity (Figure 2a). The Figure 2 shows the following variables: Rainfall (Figure 2a), D.O. (Figure 2b), Temperature (Figure 2c), Total N (Figure 2d), Depth (Figure 2e), Total P (Figure 2f), pH (Figure 2g),Chlorophyll (Figure 2h), Conductivity (Figure 2i), and Trophic State Index (Figure 2j).
In the Sapucai River arm of the Furnas hydroelectric station reservoir, the water was well oxygenated and the highest D.O. content (8.52 mg.L-1) was during June and the lowest (5.97 μg.L-1) in February (Figure 2b). Water temperature varied from month to month, with the highest value (27.0 °C) recorded in March and lowest (20.8 °C) in June (Figure 2c). The pH varied between slightly acid and alkaline, remaining close to neutral throughout the study period (Figure 2g). Regarding the nutrients, the greatest concentrations of nitrogen and phosphorous were recorded during the rainy period, between October and January (Figures d, f). Chlorophyll a values were relatively low, except in January, when a peak of 79.5 μg.L-1 was registered. During the rest of the study period, little the variation was observed: average chlorophyll a value was 6.6 ± 6.5 μg.L-1 (Figure 2h). Electrical conductivity was relatively low, with a narrow range of variation, between 14.9 μS.cm-1 (January) and 28.9 μS.cm-1 (November) (Figure 2i).
On the basis of the TSI employed, the Sapucaí arm of Furnas reservoir was classified as oligotrophic, except in January, when it was mesotrophic (Figure 2j).
Zooplankton community in the Sapucaí arm of the Furnas reservoir was constituted by Rotifera, Cladocera and Copepoda and, occasionally, by other taxa, such as Ostracoda, Turbellaria, the dipteran's larvae Chaoborus brasiliensis and Protozoa, mainly the tecamoebae Arcella sp. and Difflugia sp. Rotifers were dominant in 5 of the 12 months, while copepods dominated during 6 months. The least abundant group was Cladocera, which was found in low densities as compared with rotifers and copepods, except during February, when their numbers exceeded those of the other two groups (Tables 1, 2 and 3).
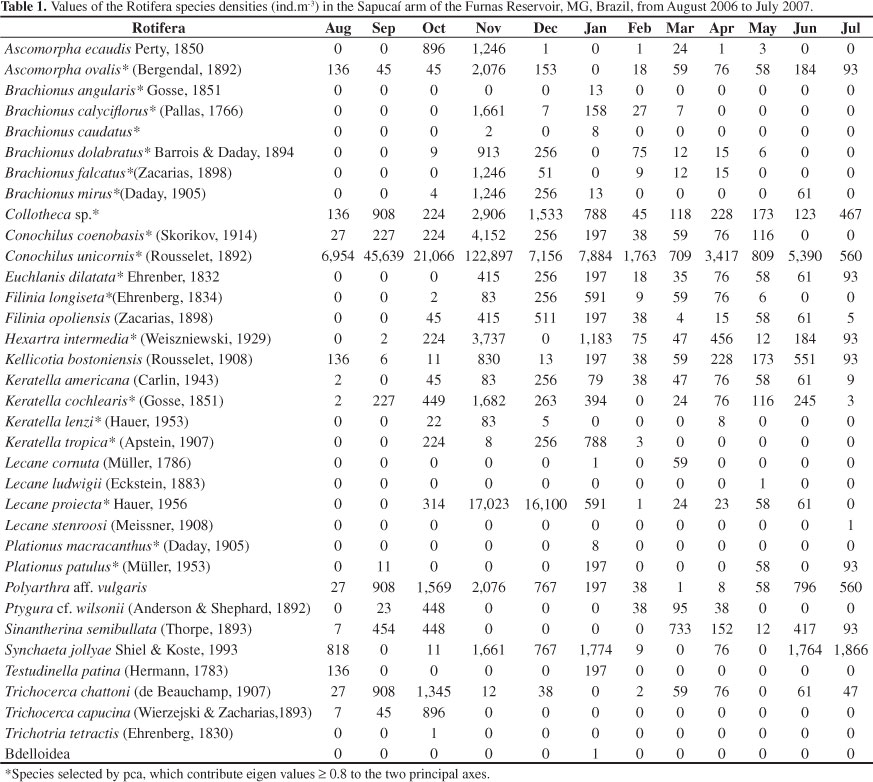
Rotifera was represented by 35 taxa. The dominant species were Ascomorpha ecaudis, A. ovalis, Brachionus dolabratus, Collotheca sp., Conochilus coenobasis, C. unicornis, Filinia longiseta, F. opoliensis, Hexarthra intermedia, Kellicottia bostoniensis, Keratella americana, K. cochlearis, Lecane proiecta, Polyarthra aff. vulgaris and Sinantherina semibullata (Table 2). The highest density of rotifers (166,854 ind.m-3) was recorded in November and a single species (C. unicornis) was responsible for most of this peak, contributing 122,897 ind.m-3 (Figure 3).
Crustaceans with high abundances, comparable to rotifer densities and possibly competing with rotifer populations in this part of the reservoir were mostly the species Bosmina freyi, B. hagmanni, B. tubicen, Ceriodaphnia cornuta cornuta, C. cornuta intermedia, C. cornuta rigaudi, C. silvestrii; Diaphanosoma birgei, D. brevireme, D. fluviatile and D. spinulosum; Moina minuta; Notodiaptomus cearensis; N. spinuliferus; calanoid nauplii and copepodites and cyclopoid nauplii (Table 3). Potential predators of rotifers were represented by juvenile and adult stages of cyclopoid copepods Thermocyclops minutus, T. decipiens and Mesocyclops ogunnus, larvae of Chaoborus brasiliensis, the rotifer Asplanchna sieboldi and turbellarians (Table 3).
When the Monte Carlo test was applied to the sets of environmental factors, it was found that chlorophyll a, and crustacean competitors (cladocerans of family Moinidae, predatory copepods Thermocyclops decipiens and Mesocyclops ogunnus) had significant effects on the rotifer populations (Table 4).
Redundancy Analysis (RDA) applied to rotifer populations, and physical, chemical and biological (chlorophyll a) variables indicated that the first two ordination axes explained 69% of the total variance in the results. Among the explanatory variables, D.O. and electrical conductivity showed the least influence on rotifer numbers. The ordination chart (Figure 4a) showed that chlorophyll a was the environmental factor that most strongly influenced the rotifers (p < 0.05) (Table 4). The chart also showed that the species Brachionus angularis, B. caudatus, Plationus macracanthus, P. patulus and Filinia longiseta occurred in the rainy period and increased collinearly with phytoplankton biomass.
The RDA between Rotifera populations and potential competitor species explained 70.9% of total variance. Representatives of Chydoridae and Sididae families of Cladocera had the least influence on rotifer densities (Figure 4b). Ordination diagram indicated that representatives of Moinidae had the strongest influence upon the rotifer populations (p < 0.05) (Table 4), and showed that Conochilus coenobasis, Brachionus falcatus, Lecane proiecta, Keratella tropica and B. dolabratus occurred in the rainy period and had positive colinearity with the Moinidae cladocerans.
RDA between rotifers and predator species explained 86.2% of total variability accumulated in the two first ordination axes. Triplot ordination analysis indicated that the cyclopoid copepod Thermocyclops minutus was the predatory species with the least influence over the Rotifera populations, although it occurred in high densities throughout the whole study period. It further revealed that the species Thermocyclops decipiens (p < 0.05) and Mesocyclops ogunnus (p < 0.001) (Figure 4c) constituted the most important predators controlling rotifer populations. Thus, for the dry period there is a negative collinearity between T. decipiens densities and Euchlanis dilatata, Keratella cochlearis, B. calyciflorus, F. longiseta, H. intermedia and K. tropica densities. Conversely, there was a positive linear correlation with M. ogunnus, a potential predator of most of the Rotifera populations.
According to these results, obtained from the co-occurrence analysis, it could be inferred that competition among Rotifera populations occurred only from November to January (rainy period) (p < 0.05), with a C-score (0.18) higher than that randomly expected (0.14).
Discussion
The two well-defined climatic seasons that occur in the studied area, a dry winter and a rainy summer, resulted in significant changes in the abiotic and biotic variables observed in the reservoir arm formed by the Sapucaí River.
A majority of the rotifer species (22 out of 35) was recorded at high densities at the start of the rainy season while the occurrence of certain species - Brachionus angularis, B. calyciflorus, B. caudatus, Keratella cochlearis var. tecta, K. tropica and Plationus patulus - was entirely restricted to this period. Conversely, Ptygura cf. wilsonii and Sinantherina semibullata, species of the family Flosculariidae, occurred not in the rainy season, but mainly in the transition from the rainy to the dry season and during the dry period.
The pH, electrical conductivity and D.O. concentrations probably had little effect on the fluctuations of the rotifer populations, since the whole ranges of variation of the variables observed were inside the range considered adequate for most zooplankton species (Sipaúba-Tavares & Rocha 2003). Eventhough the electrical conductivity did not contribute significantly to the correlations revealed by the RDA, the highest value recorded (28.9 μS.cm-1) coincided with the peak in the total rotifer densities, in November. Toledo et al. (2003) have pointed out that moderate electrical conductivities (around 40 μS.cm-1) can favor the growth and even the dominance of Rotifera in tropical waters.
Light penetration in the water column was limited in Furnas reservoir. This is a common fact in tropical reservoirs because heavy rainfall usually carries large quantities of inorganic matter from neighboring lands into streams flowing to the reservoirs (Payne 1986).
Positive correlation was found between rotifer density and chlorophyll a concentrations, evidencing the importance of food, noticeably for Brachionus caudatus. Food availability is a biotic factor frequently related to rotifer population densities and diversity (Bonecker et al. 2005, Deveter & Sed´a 2005, Duggan et al. 2001).
In the Sapucaí arm of the reservoir, cladocerans were represented chiefly by the genera Bosmina, Ceriodaphnia, Diaphanosoma and Moina. As a rule, both rotifers and cladocerans prefer to filter algal cells lesser than 50 μm of size (MacIsaac & Gilbert 1989, Jaramillo-Londiño & Pinto-Coelho 2010). Kirk (1997) suggests that when algal biomass acts as a limiting factor, rotifers could be at disadvantage in the competition for food, since they are less resistant to starvation. Hurtado-Bocanegra et al. (2002) found that the population growth of B. patulus was negatively influenced by the presence of Ceriodaphnia dubia and M. macrocopa and that there is a complex interaction between rotifers and cladocerans.
Results of the RDA indicated positive collinearity between Moinidae density (mainly Moina minuta) and that of rotifers Conochilus coenobasis, Brachionus falcatus, Lecane proiecta, Keratella tropica and B. dolabratus. In general survival strategies of Moina species are similar to those of rotifers, which have high rates of population growth and short life cycles (Nandini & Sarma 2000). In the present study Moina minuta population probably had no prejudicial effect on rotifers, since the latter were found at far higher densities (around 800 - fold higher) throughout the period of study. Even though clearance rates of Moina are around 25 times greater than rotifer clearance rates - (235 μL ind.-1/hour- for Moina micrura (Macedo & Pinto-Coelho 2000), and 9 μL ind.-1/hour - for Keratella cochlearis, Polyarthra vulgaris and P. dolychoptera (Bogdan & Gilbert 1982).
Analysis of the co-occurrences indicated that in the rainy period (November to January), competitive interactions were strong among rotifer populations (p < 0.001). This fact is consistent with the high species diversity and high densities of rotifers observed in the Sapucaí arm during the period of study. In natural environments herbivore communities can compete strongly by limiting resources leading to coexistence or exclusion of species (DeMott & Kerfoot 1982).
Although the food niches of rotifers are more specialized than those of cladocerans (Bogdan & Gilbert 1982), limiting resources can lead to inter-specific competition depending on rotifer sizes, food concentration and the initial densities of the competing species (Sarma et al. 2008).
Predators with most of rotifers observed in Furnas reservoir were the copepodites and adults of Cyclopoida (Mesocyclops ogunnus, Thermocyclops decipiens and T. minutus), the larvae of the dipteran Chaoborus brasiliensis, the rotifer Asplanchna sieboldi and turbellarians. In the rainy season, Mesocyclops ogunnus numbers showed significant positive correlation (p < 0.001) with most rotifers and, due to the low Mesocyclops densities, this copepod probably had no negative effect on the rotifer populations. In the dry season, adults of Thermocyclops decipiens were recorded at high densities and showed negative collinearity (p < 0.05) with the rotifer species Euchlanis dilatata, Keratella cochlearis, Brachionus calyciflorus, Filinia longiseta, Hexarthra intermedia and K. tropica, according to RDA results.
Predation pressure exerted by Thermocyclops decipiens probably determined the decrease in rotifer densities in this reservoir arm, in the dry period. The highly-developed ability of species of the suborder Cyclopoida to seize their prey among the zooplankton is already well-established (Plaßmann et al. 1997, Williamson, 1983, 1984, Matsumura-Tundisi et al. 1997, Dieguez & Gilbert 2002). Representatives from many taxa are reported to feed on rotifers. There are good quantitative data on predation on rotifers by both, predaceous Rotifera and Cyclopoida copepods (Williamson & Gilbert 1980, Stemberger & Evans 1984). Aditionally, the limited information available on other predatory invertebrate taxa present in Furnas reservoir zooplankton suggests that rotifer production may contribute extensively to their diets.
Among the environmental factors, food availability as indicated by algal biomass, had an important role on rotifer abundances during the rainy period, but inter-rotifer competition was also significant in these months. On the other hand, it was probably that predation by the cyclopoid copepod Thermocyclops decipiens determined the structure and dynamics of the rotifer populations in the Sapucaí arm of Furnas reservoir during the dry period.
Acknowledgments
Authors are grateful to the Hydrobiology and the Fish farming Unit of FURNAS Centrais Elétricas SA for allowing access to their facilities; to the Research and Development Program of Aneel for financial assistance to research, and to the Brazilian National Research Council (CNPq) for the student award. We also thank Timothy C. Roberts by the English corrections.
Received 27/05/2010
Revised 20/11/2010
Accepted 29/11/2010
- ALMEIDA, V.L.S., DANTAS, Ê.W., MELO-JÚNIOR, M., BITTENCOURT-OLIVEIRA, M.C. & MOURA, A.N. 2009. Zooplanktonic community of six reservoirs in northeast Brasil. Braz. J. Biol. 69(1):57-65.
- AOYAGUI, A.S.M. & BONECKER, C.C. 2004. Rotifers in different environments of the upper Parana River floodplain (Brazil): richness, abundance and the relationship to connectivity. Hydrobiologia 522:281-290.
- BOGDAN, K.G. & GILBERT, J.J. 1982. Seasonal patterns of feeding by natural populations of Keratella, Polyarthra and Bosmina Clearance rates, selectivity and contributions to community grazing. Limnol. Oceanogr. 27:918-934.
- BONECKER, C.C. & LANSAC-TÔHA, F.A. 1996. Community structure of rotifers in two environments of the upper River Paraná floodplain (MS)-Brazil. Hydrobiologia 325:37-150.
- BONECKER, C.C. DA COSTA, C., LUIZ VELHO, L. & LANSAC-TÔHA, F. 2005. Diversity and abundance of the planktonic rotifers in different environments of the Upper Paraná River floodplain (Paraná State - Mato Grosso do Sul State, Brazil). In Rotifera X - Rotifer research: trends, new tools and recent advances (A. Herzig, R.D. Gulati, C.D. Jersabek & L. May, eds.), p405-414.
- BORGES, M.G. & PEDROZO, C.S. 2009. Zooplankton (Cladocera, Copepoda and Rotifera) richness, diversity and abundance variations in the Jacuí Delta, RS, Brazil, in response to the fluviometric level. Acta Limnol. Bras. 21(1):101-110.
- CARLSON, R. E. 1977. A trophic state index for lakes. Limnol Oceanogr. 22(2):261-269.
- CHESSON, P. L. 1986. Environmental variation and the coexistence of species. In: Harper and Row. (J. Diamond & T. Case, ed.). Commuity Ecol., New York, p.240-256.
- COELHO-BOTELHO, M.J. 2004. Dinâmica da comunidade zooplanctônica e sua relação com o grau de trofia em reservatório. Bol. CETESB. www.cetesb.sp.gov.br (último acesso em 16/08/2009).
- CORGOSINHO, P.H.C. & PINTO-COELHO, R.M. 2006. Zooplankton biomass, abundance and allometric patterns along an eutrophic gradient at Furnas Reservoir (Minas Gerais, Brazil). Acta Limnol. Brasil. 182:213-224.
- DEMOTT, W.R. & KERFOOT, W.C. 1982. Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecology 63:1949-1966.
- DEVETTER, M. & SED'A, J. 2005. Decline of clear-water rotifer populations in a reservoir: the role of limitation resources. Hydrobiologia 546:509-518.
- DIEGUEZ, M.C. & GILBERT, J.J. 2002. Suppression of the rotifer Polyarthra remata by the omnivorous copepod Tropocyclops extensus: predation or competition. J. Plankton Res. 24(4):359-369.
- DUGGAN, I.C., GREEN, J.D., SHIEL, R.J. 2001. Distribution of rotifers in North Island, New Zealand, and their potential use as bioindicators of lake trophic state. Hydrobiologia 446-447:155-164.
- EDMONDSON, W.T. 1959. Freshwater Biol. 2. ed. Wiley and Sons, New York, 1248p.
- ELMOOR-LOUREIRO, L.M.A. 1997. A Manual for the identification of freshwater cladocerans in Brazil (in portuguese). Brasília, Universa, 155p.
- GOLTERMAN, H.L., CLYMO, R.S. & OHNSTAD, M.A.M. 1978. Methods for physical and chemical analysis of freshwaters. Int. Biol. Program., London, 213p.
- GOTELLI, N.J. & ENTSMINGER, G.L. 2001. EcoSim: null models software for ecology. Acquired Intelligence Inc. and Kesey-Bear. Jericho, VT 05465. http://garyentsminger.com/ecosim
- HURTADO-BOCANEGRA, M.D., NANDINI, S. & SARMA, S.S.S. 2002. Combined effects of food level and innoculation density on competition between Brachionus patulus (Rotifera) and the cladocerans Ceriodaphnia dubia and Moina macrocopa. Hydrobiologia 468:13-22.
- JARAMILLO-LONDIÑO, J.C & PINTO-COELHO, R.M. 2010. Interaction between Hexarthra and Bosmina. J. Plankton Res. 2010.
- KIRK, K.L. 1997. Life-history responses to variable environments: starvation and reproduction in planktonic rotifers. Ecology 78:434-441.
- KOSTE, W. 1978. Rotatoria Die Rodertiere Mitteleuropas begründet von Max Voigt-Monogononta. 2. Auflage neubearbeitet von Walter Koste. Gebrüder Borntraeger, 1, 238p.
- KOSTE, W. & ROBERTSON, B. 1990. Taxonomic studies of the Rotifera from shallow waters on the Island of Maracá, Roraima, Brazil. Amazoniana Kiel 11(2):185-200.
- LEHMAN, J.T. 1991. Interacting growth and loss rates: the balance of top-down and bottom-up controls in plankton communities. Limnol. Oceanogr. 36(8):1546-1554.
- LIMA, A.F., LANSAC-TÔHA, F.A & BONECKER, C.C. 1996. Zooplankton in the Floodplains of a Tributary to the Paraná River in Mato Grosso do Sul, Brazil. Stud. Neotrop. Fauna Environ. 31(2):112-116.
- LORENZEN, C.J. 1967. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnol. Oceanogr. 12:343-346.
- MACEDO, C.F. & PINTO-COELHO, R.M. 2000. Efeitos das algas Ankistrodesmus gracilis e Scenedesmus quadricauda no crescimento e no índice lipídico de Daphnia laevis e Moina micrura. Acta Sci. 22(2):397-401.
- MACISAAC, H.J. & GILBERT, J.J. 1989. Competition between rotifers and cladocerans of different body sizes. Oecologia 81:95-301.
- MACKERETH, J.F.H., HERON, J. & TALLING, J.F. 1978. Water analysis: some revised methods for limnologists. Fresh. Biol. Ass. 36:121.
- MARTÍNEZ, J.C.C., CANESIN, A. & BONECKER, C.C. 2000. Species composition of rotifers in different habitats of an artificial lake, Mato Grosso do Sul State, Brazil. Acta Sci. 22(2):343-346.
- MATSUMURA-TUNDISI, T. 1986. Latitudinal distribution of Calanoida Copepods in freshwater aquatic systems of Brazil. Braz. J. Biol. 43(3):527-553.
- MATSUMURA-TUNDISI, T., ROCHA, O. & TUNDISI, J.G. 1997. Carbon uptake by Scolodiaptomus corderoi and Thermocyclops minutus feeding on different size fractions of phytoplankton from Lake Dom Helvécio. In Limnological Studies on the Rio Doce Valley Lakes, Brazil (J.G. Tundisi & Y. Saijo, ed.). Brazilian Academy of Sciences, University of São Paulo School of Engineering at S. Carlos and Center for Water Resources and Applied Ecology, São Paulo, p.275-284.
- NANDINI, S. & SARMA, S.S.S. 2000. Lifetable demography of four cladoceran species in relation to algal food (Chlorella vulgaris) density. Hydrobioly 435:117-126.
- OLIVEIRA-NETO, A.L. & MORENO, I.H. 1999. Filo Rotifera. In Biodiversidade do Estado de São Paulo, Brasil. Invertebrados de Água Doce. (D. Ismael, W.C. Valente, T. Matsumura-Tundisi, O. Rocha, org.). São Paulo: FAPESP, 4, p.39-52.
- PAYNE, R. 1986. The ecology tropical lakes and rivers. New York: John Wiley & Sons, 301p.
- PLAßMANN, T., MAIER, G. & STICH, H.B. 1997. Predation impact of Cyclops vicinus on the rotifer community in Lake Constance in spring. J. Plankton Res. 18:1069-1079.
- REID, J.W. 1985. Identification key and list of references for the South-American free living inland species of order Cyclopoida (Crustacea, Copepoda). B. Zool. Univ. S. Paulo, 9, p.17-143.
- ROCHA, O. & MATSUMURA-TUNDISI, T. 1976. Atlas of the zooplankton of Broa Reservoir, São Carlos-SP, Ed. UFSCar, 68p.
- SARMA, S.S.S., FRANCO-TÉLLEZ, J.L. & NANDINI, S. 2008. Efecto de la concentración de algas (Chlorella vulgaris) y la densidad de inoculación sobre la competencia entre tres Brachionidae (Rotifera: Monogononta) planctônicos. Hidrobiológica 18(1):123-132.
- SEGERS, H. 1995. Rotifera. The Lecanidae (Monogononta) Guides to the identification of the microinvertebrates of the continental waters of the world. SPB Academics, Amsterdam, 2, 226p.
- SENDACZ, S.; CALEFFI, S. & SANTOS-SOARES, J. 2006. Zooplankton biomass of reservoirs in different trophic conditions in the state of São Paulo, Brazil. Braz. J. Biol. 66(1B):337-350.
- SIPAÚBA-TAVARES, L.H. & ROCHA, O. 2003. Production of plankton (phytoplankton and zooplankton) for feeding aquatic organisms. Rima, São Carlos, 106p. In portuguese.
- SMIRNOV, N.N. 1996. Cladocera: The Chydorinae and Saycinae (Chydoridae) on the world. In Guides to the identification of the microinvertebrates of the continental waters of the world (H.J. Dumont, ed.) SPB Academic, Amsterdam, 197p.
- STEMBERGER, R.S. & EVANS, M.E. 1984. Rotifer seasonal succession and copepod predation in Lake Michigan. Great Lakes Res. 10:417-428.
- STREBLE, H. & KRAUTER, D. 1987. Atlas de los microorganismos de agua dulce: la vida en una gota de agua. Libro de clasificación con 1700 ilustraciones. Omega, Barcelona, 371p.
- TER BRAAK, C.J.F. & MILAUER, P. 2002. Canoco reference manual and CanoDraw for Windows user's guide: software for canonical community ordination. Microcomputer Power, Ithaca, NY.
- TOLEDO, A.P., TALARICO, M., CHINEZ, S.J. & AGUDO, E.G. 1983. The use of simplified models to evaluate the process of eutrophication in tropical lakes and reservoirs. In: 12th Brazilian Congress in Sanitary Engineering and Environment. Cetesb, São Paulo, p.1-34. In portuguese.
- TOLEDO, J.J., CASTRO, J.G.D., SANTOS, K.F., FARIAS, R.A., HACON, S. & SMERMANN, W. 2003. Evaluation of environmental impacts caused by the effluents of fish-farming tanks in the Pisciculcute Station at Alta Floresta - MT. Rev. Prog. Ciên Agr-Amb, Alta Floresta 2(1):13-31. In portuguese.
- TUNDISI, JG., MATSUMURA-TUNDISI, T., HENRY, R. & ROCHA, O. 1988. Comparison of the trophic state of 23 reservoirs of São Paulo State: Eutrophication and management. In Limnologia e Manejo de represas (J.G. Tundisi, ed.). p.165-204.
- WILLIAMSON, C.E. & GILBERT, J.J. 1980. Variation among zooplankton predators: the potential of Asplanchna, Mesocyclops, and Cyclops to attack, capture, and eat various rotifer prey. In Evolution and Ecology of Zooplankton Communities (W.C. Kerfoot, ed.). The University Press of New England, Hanover, p.509-517.
- WILLIAMSON, C.E. 1983. Invertebrate predation on planktonic rotifers. Hydrobiologia 104(1):385-396.
- WILLIAMSON, C.E. 1984. Laboratory and field experiments on the feeding ecology of the cydopoid copepod, Mesocyclops edax Freshwater Biol. 14:575-585.
Publication Dates
-
Publication in this collection
29 July 2011 -
Date of issue
Dec 2010
History
-
Accepted
29 Nov 2010 -
Reviewed
20 Nov 2010 -
Received
27 May 2010