Abstracts
Shipwrecks are considered artificial reef environments - structures immersed in aquatic environments (especially marine ones) that provide fauna with shelter, hard substrates, food and nursery areas. This study aimed to survey the benthic animal biodiversity of the Pirapama shipwreck, located 23 m deep and six miles off Recife harbor. From 2001 to 2007, species were observed, photographed and collected through scuba diving. The material was later sorted and identified in the laboratory. A total of 76 sessile and sedentary animal taxa were recorded belonging to the following phyla: Porifera (Demospongiae and Calcarea), Cnidaria (Hydrozoa and Anthozoa), Mollusca (Bivalvia and Gastropoda), Annelida (Polychaeta), Arthropoda (Cirripedia), Bryozoa (Gymnolaemata), Echinodermata (Asteroidea and Echinoidea), and Chordata (Ascidiacea). The greatest richness was for Porifera and Bryozoa - 13 listed species for each. Eleven new occurrences were recorded for the state of Pernambuco, the hydroid Halopteris polymorpha and ten bryozoan species, one of them being the first record for Brazil (Scrupocellaria curacaoensis). The Pirapama's biodiversity was considered typical when compared to other shipwrecks that have been studied around the world.
artificial reefs; biofouling; benthic fauna inventory; marine invertebrates; biodiversity
Naufrágios são classificados como ambientes recifais artificiais, estruturas imersas em ecossistemas aquáticos (principalmente marinhos) que fornecem abrigo, substratos consolidados, áreas de crescimento, alimentação e de berçário. Este estudo objetivou pesquisar a biodiversidade bentônica animal do Naufrágio Pirapama, localizado a 23 m de profundidade e a seis milhas do Porto de Recife. Entre 2001 e 2007 mergulhos autônomos foram realizados para coletar, observar e fotografar as espécies. No laboratório, o material foi posteriormente classificado e identificado. Um total de 76 táxons foi registrado para a fauna séssil e sedentária, pertencentes aos seguintes filos: Porifera (Demospongiae e Calcarea), Cnidaria (Hydrozoa e Anthozoa), Mollusca (Bivalvia e Gastropoda), Annelida (Polychaeta), Arthropoda (Cirripedia), Bryozoa (Gymnolaemata), Echinodermata (Asteroidea e Echinoidea), e Chordata (Ascidiacea). A maior quantidade de espécies foi de Porifera e Bryozoa, com 13 espécies listadas para cada um. Onze novas ocorrências foram registradas para o Estado de Pernambuco, o hidróide Halopteris polymorpha e dez espécies de briozoários, uma deles sendo o primeiro registro para o Brasil (Scrupocellaria curacaoensis). A biodiversidade do Pirapama foi considerada típica quando comparada com a de outros naufrágios que foram estudados no mundo.
recifes artificiais; biofouling; inventário da fauna bentônica; invertebrados marinhos; biodiversidade
ARTIGOS
Sessile and sedentary macrofauna from the Pirapama Shipwreck, Pernambuco, Brazil
Macrofauna séssil e sedentária do Naufrágio Pirapama, Pernambuco, Brasil
Simone Maria de Albuquerque LiraI, * * Corresponding author: Simone Maria de Albuquerque Lira, e-mail: simonealira@gmail.com ; Cristiane Maria Rocha FarrapeiraII; Fernanda Maria Duarte AmaralII; Carla Alecrim Colaço RamosII
IDepartamento de Oceanografia, Universidade Federal de Pernambuco - UFPE, Av. Prof. Moraes Rego, n. 1235, CEP 50670-901, Cidade Universitária, Recife, PE, Brasil
IIÁrea de Zoologia, Departamento de Biologia, Universidade Federal Rural de Pernambuco - UFRPE, Rua Dom Manoel de Medeiros, s/n, CEP 52171-900, Dois Irmãos, Recife, PE, Brasil
ABSTRACT
Shipwrecks are considered artificial reef environments - structures immersed in aquatic environments (especially marine ones) that provide fauna with shelter, hard substrates, food and nursery areas. This study aimed to survey the benthic animal biodiversity of the Pirapama shipwreck, located 23 m deep and six miles off Recife harbor. From 2001 to 2007, species were observed, photographed and collected through scuba diving. The material was later sorted and identified in the laboratory. A total of 76 sessile and sedentary animal taxa were recorded belonging to the following phyla: Porifera (Demospongiae and Calcarea), Cnidaria (Hydrozoa and Anthozoa), Mollusca (Bivalvia and Gastropoda), Annelida (Polychaeta), Arthropoda (Cirripedia), Bryozoa (Gymnolaemata), Echinodermata (Asteroidea and Echinoidea), and Chordata (Ascidiacea). The greatest richness was for Porifera and Bryozoa - 13 listed species for each. Eleven new occurrences were recorded for the state of Pernambuco, the hydroid Halopteris polymorpha and ten bryozoan species, one of them being the first record for Brazil (Scrupocellaria curacaoensis). The Pirapama's biodiversity was considered typical when compared to other shipwrecks that have been studied around the world.
Keywords: artificial reefs, biofouling, benthic fauna inventory, marine invertebrates, biodiversity.
RESUMO
Naufrágios são classificados como ambientes recifais artificiais, estruturas imersas em ecossistemas aquáticos (principalmente marinhos) que fornecem abrigo, substratos consolidados, áreas de crescimento, alimentação e de berçário. Este estudo objetivou pesquisar a biodiversidade bentônica animal do Naufrágio Pirapama, localizado a 23 m de profundidade e a seis milhas do Porto de Recife. Entre 2001 e 2007 mergulhos autônomos foram realizados para coletar, observar e fotografar as espécies. No laboratório, o material foi posteriormente classificado e identificado. Um total de 76 táxons foi registrado para a fauna séssil e sedentária, pertencentes aos seguintes filos: Porifera (Demospongiae e Calcarea), Cnidaria (Hydrozoa e Anthozoa), Mollusca (Bivalvia e Gastropoda), Annelida (Polychaeta), Arthropoda (Cirripedia), Bryozoa (Gymnolaemata), Echinodermata (Asteroidea e Echinoidea), e Chordata (Ascidiacea). A maior quantidade de espécies foi de Porifera e Bryozoa, com 13 espécies listadas para cada um. Onze novas ocorrências foram registradas para o Estado de Pernambuco, o hidróide Halopteris polymorpha e dez espécies de briozoários, uma deles sendo o primeiro registro para o Brasil (Scrupocellaria curacaoensis). A biodiversidade do Pirapama foi considerada típica quando comparada com a de outros naufrágios que foram estudados no mundo.
Palavras-chave: recifes artificiais, biofouling, inventário da fauna bentônica, invertebrados marinhos, biodiversidade.
Introduction
The concept of "artificial reef" defines a group of activities that aim to remodel the marine ecosystem by offering new habitats (Seaman 2000). They are also commonly used in underwater leisure activities; the development of local marine life promoted by such submersed structures makes these environments favorable sites for recreational diving, for example (Dowling & Nichol 2001). Oceanic platforms, docks, dikes, jetties and sea walls are some of the environments that fit this definition and that essentially function as artificial rocky coasts (Pickering et al. 1998). Shipwrecks are also classified as artificial reefs and are structures that have been accidentally or deliberately sunk in aquatic environments, especially marine ones. Similarly to natural reef environments, they provide substrate for benthic fauna, shelter from predation and tidal currents, growth and food areas, nursery space for fauna, and recruitment habitats for individuals that would otherwise lose themselves from the rest of the population (Hixon & Beets 1989, Pickering & Whitmarsh 1997, Pickering et al. 1998, Amaral et al. 2006, Krohling et al. 2006, Walker et al. 2007). Additionally, there is high biodiversity and increased connectivity between natural and artificial reefs, the former which occupy less than 0.25% of the ocean (Knowlton 2008).
In these areas the biological encrustations observed (referred to as "fouling") include sessile fauna and flora communities that attach themselves to hard substrates (epifauna and epiflora), as well as boring species (infauna) or free-living species that hide in crevices (sedentary) (Whoi 1952, Floerl et al. 2004). The benthic fauna carries out an essential ecological role in aquatic ecosystems, since most of these organisms participate in organic matter decomposition and in cycling the substrate's nutrients. Thus, such fauna occupies an important position within the food web and is the main food item of many nektonic animals (Nybakken 1993, Snelgrove 1998).
Researches related to the benthic organisms found in different submerged artificial structures have been carried out in several regions of the world (Woodhead & Jacobson 1985, Wendt et al. 1989, Bull & Kendall 1994, Zintzen et al. 2006, 2008). In Brazil, pioneering studies with artificial reefs began in 1985, in Rio de Janeiro, as an attempt to prevent predatory fishing with bottom trawls used to capture shrimp (Santos & Passavante 2007). From the 1990's on, other states initiated partnerships with universities and nongovernmental organizations in order to develop projects to implement artificial reefs on their continental platforms. The submerged structures used vary from tires to ship hulls and aim to act as biological fish attractors (Conceição et al. 1997). Presently other studies are being carried out to test different types of artificial substrates and evaluate the recruitment and colonization of encrusting species on artificial reefs; their purpose is to determine the role of structural complexity and benthos for fish community habitats (Scheffer 2001, Zalmon & Gomes 2003, Azevedo et al. 2006, Brotto et al. 2006, Krohling et al. 2006).
Only two studies have been carried out with benthic communities associated with shipwrecks in Brazil. One of them was undertaken off the coast of Espírito Santo State (southeastern region) and focused on the Victory 8B shipwreck, which was sunk in 2003 at an average depth of 32 m (Almeida 2007). The other study was carried out at two shipwrecks with different ages - Vapor de Baixo and Servemar X - sunk in 1850 and 2002, respectively, at an average depth of 23 m at the coast of Pernambuco State (Amaral et al. 2010). The Pirapama shipwreck is part of a group of approximately 70 shipwrecks - some of which date to the 18th century, when Brazil was still a colony - which explains why the city of Recife is called "the shipwreck capital". The so-called "shipwreck park" located in this area serves an expanding underwater tourism market by providing favorable areas for visiting sunken ships (Santos & Passavante 2007, Santos et al. 2008).
Massin et al. (2002) justify that technical issues are the main reasons why this community is not better studied. Yet the fauna that inhabits structures such as shipwrecks is a part of the biological community that cannot be neglected (Zintzen et al. 2006). Thus, the aim of this study was to uncover the benthic animal biodiversity with emphasis on sessile and sedentary macrofauna of the Pirapama shipwreck.
Material and Methods
The Pirapama shipwreck was sunk as scrap in 1889 and is located six miles off Recife harbor (08º 03' 23" S and 034º 46' 58" W) and from natural hard bottom communities of coastal reef environments (Figure 1). It is presently settled on a sandy bottom at an average depth of 23 m; its iron hull - about 60 m long and 8 m wide - is in good condition and is only worn due to weathering (Carvalho 1997, 2010) (Figure 2). The deck is now partially covered by sediment, out of which some of its structures emerge vertically (Figure 3). There are two seasons in the area: a dry season (September to February) and a rainy season (March to August). Average precipitation is high and reaches approximately 2.500 mm per year. Water temperature varies from 27 to 29 ºC, while salinity averages 29 and 37, respectively, in the rainy and dry months; water transparency goes up to 23 m (Carvalho 2010, Costa et al. 2010).
The biological data was collected through a series of 43 scuba dives conducted from 2001 to 2007 by groups of two or three divers. Some species of sponges, corals and echinoderms were identified in the field. The remaining sessile and sedentary invertebrates were collected taking only a few samples per morphospecies to minimize environmental impact. Species' spatial distribution and abundance in the different habitats (subjected to direct/indirect light, sedimentation and hydrodynamism) were recorded in situ on PVC plates and complemented by photographs.
In the laboratory, the organisms were fixed in 4% formaldehyde or 70% alcohol, following specifications for each taxonomic group. The material was later sorted and classified into functional groups with the aid of a stereomicroscope and relevant bibliography or sent to specialists.
Results
The benthic macrofauna found in the Pirapama shipwreck encompassed 76 taxa distributed among eight phyla. Mollusca was the dominant phylum in terms of species richness (22%), followed by Porifera (20%) and Bryozoa (17%). The remaining groups added up to 16% (Cnidaria), 8% (Arthropoda: Cirripedia), 7% (Chordata: Ascidiacea), 5% (Echinodermata), and 5% (Annelida: Polychaeta) (Table 1).
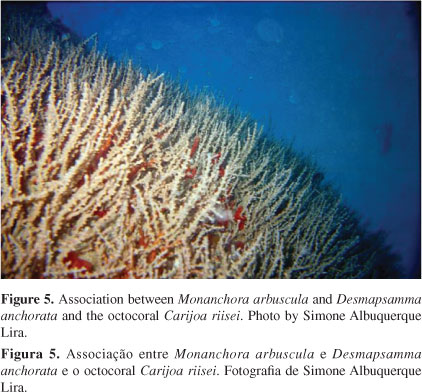
Fifteen sponge species were found (Table 1), which stood out as they covered great part of the shipwreck surfaces - especially the sheltered areas (see Figure 4). The sponges Monanchora arbuscula and Desmapsamma anchorata, associated with the octocoral Carijoa riisei, occupied almost the entire shaded area and indentations available on the shipwreck (Figure 5). This octocoral was found in several areas, especially those that were less exposed to light and sedimentation, always with epibiont organisms.
Six hydroid species were found (Table 1), all widely distributed over the shipwreck's hull. Some colonies had an epibiotic relationship with sponges, bivalves, ascidians, and algae. The most prominent organisms on the deck's horizontal surface were the sponges, corals Montastraea cavernosa and Siderastrea stellata, and their associated fauna. This last coral species was the only one with bleached specimens in the studied period (Figure 6) and occurred in continuous extensions all over the shipwreck hull - including the bow - in areas of higher hydrodynamism. The corals Astrangia solitaria, Montastraea cavernosa and Mussismilia hispida occupied a smaller area in relation to the other cnidarians, at the protected region near the stern, on surfaces exposed to light and sheltered from turbulence. The species Meandrina braziliensis was only found on the sandy areas adjacent to the shipwreck.
Molluscs constitute the most characteristic group found at the Pirapama Shipwreck, the 17 species found were represented by six gastropods and eleven bivalves (Table 1). The so-called jewel box bivalves, Chama spp., were the most numerous amongst the bivalves, and were basibionts organisms to hydroids, serpulid polychaetes, the barnacle Balanus trigonus, bryozoans (especially Hippothoa flagellum, Trypostega striatula and Nolella stipata) and ascidians. Some sessile Sabellidae and Serpulidae polychaetes were found, yet only the Christmas tree worm Spirobranchus sp. was identified to the generic level, associated with colonies of corals. One nestling polychaete was observed amongst the sessile comunity, the bearded fireworm Hermodice carunculata.
In relation to the Cirripedia, Amphibalanus amphitrite and A. improvisus were only found as empty shells, directly attached to the hull's lateral surfaces. The most abundant species among the barnacles were Balanus trigonus and Megabalanus tintinnabulum, found on the exposed sides of the hull and on what remains of the mast. Some specimens of Newmanella radiata and Verruca minuta were found as epibionts of M. tintinnabulum.
The arborescent bryozoan colonies were represented by Catenicella uberrima, Caulibugula hastingsae, C. dendrograpta, Scrupocellaria curacaoensis, S. maderensis and Scrupocellaria sp. They predominated on the lateral surfaces of the shipwreck's hull and on sheltered places, generally associated with other arborescent organisms, and forming tangled masses with hydroids. The encrusting bryozoans Hippothoa flagellum, Steginoporella magnilabris and Trypostega striatula along with the creeping stolonate colonies of Aetea ligulata, Beania kuglei, Buskia socialis and Nolella stipata did not seem to prefer any particular type of substrate and were found as epibionts to a great variety of calcareous animals such as bivalves, barnacles, and other bryozoans. Among the sampled bryozoans, the most conspicuous encrusting species was Steginoporella magnilabris. It stood out due to the large amount of colonies and was found on several specimens of the barnacles Balanus trigonus and Newmanella radiata as well as on Chamidae bivalves.
Four echinoderm species were recorded. A small number of starfish - Oreaster reticulatus, Linckia guildingii and Astropecten sp. - were observed. The sea urchin Diadema antillarum was recorded as the most abundant.
Colonies of the ascidians Didemnum sp., Lissoclinum sp. and Aplidium lobatum were observed growing over other animal species, especially barnacles and bivalves. Two solitary species were also recorded, Ascidia sp. and Microcosmus exasperatus, this last one commonly found in the shipwreck indentations.
Discussion
Solely considering the sessile and macrofauna species reported for the Pirapama, as well as the statement of Bortone et al. (2000) that marine artificial reef assemblages may have well over 50 different species, the faunal diversity of the Pirapama' shipwreck was considered typical when compared to other shipwrecks that have been studied around the world. For the Brazilian coast, Almeida (2007) recorded 31 species for the Victory 8b shipwreck in Espírito Santo State, which had been sunk at 32 m two years before the study. Amaral et al. (2010), in turn, found 57 species on two shipwrecks (Servemar X and Vapor de Baixo) off the Pernambuco coast, near the wreck studied here.
Considering other localities in the Atlantic Ocean, Wendt et al. (1989) while carrying out research with five shipwrecks located between South Carolina and Georgia (22-31 m deep and 3.5-10 years old) reported a total of 89 species; Baynes & Szmant (1989) recorded 52 macrofaunal taxa on the Biscayne shipwreck, located in Florida 20 m deep; and Zintzen et al. (2006) found 49 macrofauna species on the Birkenfels (42 m depth) and Bourrasque (16 m) shipwrecks, sunk for at least 10 years off the Belgian coast. Later, these same authors (Zintzen et al. 2008) investigated the cover, community structure and abiotic environment of nine shipwrecks from Belgian coastal waters; the vessels had been sunk for at least 40 years (3-22 m above the seabed) and a total of 90 species were found. Similarly, Hiscock et al. (2010) found 263 taxa while analyzing the community that colonized the frigate HMS Scylla' shipwreck, after five years on the seabed off the English coast. In the Indian Ocean, Dipper (1991) studied a shipwreck in the Arabian Gulf (20 m deep), and recorded 56 sessile and sedentary animals. In the Indo-Pacific Ocean, another report done by Walker et al. (2007) recorded 31 epifaunal taxa on the ex-HMAS Brisbane shipwreck, after the ship had been scuttled, which rests 27 m deep in the waters of Australia.
The differences in relation to the findings of those surveys might be explained by the great difference in the age of the shipwrecks when compared to the Pirapama, as well as distinctions in depth, distance from the coast, local diversity, and in the methods used to evaluate the fauna's diversity. For this reason, it is hard to confirm which factors determine the number of species found on this and in other studies.
Regarding the distribution of species found in this study and their ecological preferences, all sponge species cited are common for the Brazilian coast (Muricy & Hajdu 2006). On two nearby shipwrecks, Amaral et al. (2010) observed that Porifera was the main group in terms of number of species and space occupation, with seven coincident species: Aplysina fulva, Chondrilla nucula, Cliona cf. delitrix, Desmapsamma anchorata, Dysidea sp., Ircinia strobilina, Monanchora arbuscula, and Mycale microsigmatosa. Studying the Biscayne shipwreck, Baynes & Szmant (1989) observed that approximately half of the living cover consisted of sponges (mostly encrusting forms). From the compiled list, these authors found four species also reported from the Pirapama shipwreck: A. fistularis, D. anchorata, Monanchora arbuscula, and Scopalina ruetzleri. These species were found growing on the deck and on most of the side sections of the shipwreck. Chondrilla nucula was also recorded on artificial reefs at shallow depths (2.3-2.4 m) in Florida (Cummings 1994).
Commensalism among Carijoa riisei and the sponges Monanchora arbuscula and Desmapsamma anchorata may even be considered a pattern among these species, whereas this same association was also observed by Mothes et al. (2003), Cerrano et al. (2006), and Amaral et al. (2010). Calcinai et al. (2004) had considered this a symbiotic association, with the two partners supporting each other by giving rise to a more rigid structure: the sponge grows vertically, stressing its own growth strategies and therefore avoiding competition for space; in return, its covering protects the octocoral from predation.
According to Bayer (1961), the way Carijoa riisei grows, by creeping stolons with an arborescent structure, is an ideal structure for epibiotic association; this fact was also verified by Neves et al. (2007) when investigating the ophiuroid epibionts of this octocoral present on a seawall in Pernambuco. This octocoral occurs throughout the entire occidental Atlantic Ocean, from Florida (USA) to the Brazilian State of Santa Catarina (Silva & Pérez 2002) and is cited as common on natural substrata of Pernambuco (Laborel 1969, Pérez 2002, Neves et al. 2007). It has also been reported on other artificial substrata, such as shipwrecks (Baynes & Szmant 1989, Wagner et al. 2009, Amaral et al. 2010), oil platforms (Bull & Kendall Jr. 1994), artificial reefs (Cummings 1994) and integrating the fouling community of harbors, usually on pier pilings that are not exposed to direct sunlight (DeFelice et al. 2001).
With the exception of Halopteris polymorpha, with the nearest occurrence in the Fernando de Noronha Archipelago (Amaral et al. 2009a) and the State of Bahia (Kelmo et al. 2003), all remaining hydroid species compiled had already been mentioned for Pernambuco by Mayal et al. (2002). The hydroids Macrorhynchia philippina and Sertularella diaphana were also recorded on Vapor de Baixo shipwreck by Amaral et al. (2010) in the Pernambuco coast. The coral species had all been previously listed by Laborel (1969) for the coast of Pernambuco. It is worth mentioning that only the massive starlet coral Siderastrea stellata was found on the Vapor de Baixo shipwreck for the coast of this state - an equally old wreck in Pernambuco (Amaral et al. 2010). Wendt et al. (1989), in their study of sunken vessels in South Carolina, found that stony and soft corals comprised a much greater proportion of the total biomass on older shipwrecks (8-10 years) than on younger ones (3.5-4.5 years). Perkol-Finkel & Benayahu (2004), while studying unplanned vertical artificial reefs in Eilat (Red Sea), found that soft corals (mainly Nephtheidae) accounted for up to 90% of the total living coverage, which was attributed to physical and biological features associated of the artificial reefs.
In relation to molluscs records, all species have been cited for Pernambuco by Tenório et al. (2000) and Rios (2009). The bivalves Pinna carnea, Hyotissa hyotis, and the gastropods Aliger gallus, Charonia variegata, and Eustrombus goliath have been cited for the Northeast region in compatible depths, on hard substrata (Rios 2009). The giant coxcomb oyster, Hyotissa hyotis, now has its second record for the Brazilian coast. It had been previously reported only for the Saint Peter and St. Paul Archipelago, at a depth of 45 m to at least 60 m (Edwards & Lubbock 1983). This species is usually found below the low tide mark and on offshore wrecks (Abbott 1974, Bieler et al. 2004), yet has also been found on a buoy investigation station 29 m deep and on a stationary oil platform (38 m) (Yan et al. 2006). It is possible that H. hiotis has a broader distribution along the Brazilian shelf - the absence of other citations might be due to the lack of studies in the depths where it tends to settle.
Another interesting record is the occurrence of Pinna carnea for the Pirapama shipwreck. It is considered an endopsammic species, which lives on sand bottoms of the Brazilian coast, from Ceará to Santa Catarina States, including the Fernando de Noronha Archipelago (Matthews & Kempf 1970, Wiggers & Magalhães 2003, Rios 2009). Its presence in the examined shipwreck was probably only possible due to the accumulation of sediments on the deck of the vessel.
As for the sessile species, emphasis should be given to species of the Chama genera, whereas they were the most numerous amongst the bivalves. They are usually found on any hard, shallow substrata (Rios 2009). Chama congregata and C. macerophylla, for example, were found on five shipwrecks located between the American states of South Carolina and Georgia, at an average depth of 25 m (Wendt et al. 1989). C. macerophylla and C. sinuosa are common foulers on oil platforms, from the intertidal to 20 m deep (Lewbel et al. 1987). These three Chama species were also recorded on the other two near shipwrecks (Amaral et al. 2010). Another bivalve species reported for the shipwreck, Pinctada imbricata, is usually found attached to corals, rocks, and mangrove roots sometimes associated to sponge in, shallow waters (Farrapeira et al. 2009, Rios 2009); however, it was also recorded on oil platforms located 6 m deep off the Rio de Janeiro coast (Ferreira et al. 2006) and on concrete blocks up to 43 m deep in Florida (Cummings 1994).
In relation to Polychaeta, only one species was identified, Hermodice carunculata, a typical predator of corals and octocorals (Souza et al. 2007). The presence of this polychaete on the shipwreck follows its ecological requirements. In Brazil, this worm has been found at the Saint Peter and St. Paul Archipelago, where it is common between 45-60 m (Edwards & Lubbock 1983, Amaral et al. 2009b); at the Fernando de Noronha Archipelago (Eston et al. 1986); and at the Rocas Atoll (Barroso & Paiva 2007). Its distribution also covers the area between the Brazilian States of Rio Grande do Norte and São Paulo, particularly in association with hard substrata, but also near the surface on flotsam or swimming freely (Barroso & Paiva 2007). Lewbel et al. (1987) mentioned it on oil platforms off Louisiana, 30 m deep.
All barnacle species had already been cited for the coast of Pernambuco by Young (1998) and Farrapeira (2009, 2010). Among the species collected and considering only those that were sampled alive, three of them are usually found on artificial substrata in shallow coastal areas. Ross (1969) and Amaral et al. (2010) mentioned Newmanella radiata as an encrusting component common on hulls of wrecked ships. Balanus trigonus has been observed in greater depths (Wirtz et al. 2006) and occupies a variety of biogenic and abiogenic substrata (Farrapeira 2010), including artificial reefs in New Zealand (Russell 1975) and Florida (Nelson et al. 1994), and shipwrecks between 20 and 30 m deep (Wendt et al. 1989, Ponti et al. 2002, Amaral et al. 2010) Megabalanus tintinnabulum has been reported up to 38 m deep on offshore oil platforms (Lewbel et al. 1987, Yan et al. 2006), and on a shipwreck that lay in 27 m of water (Walker et al. 2007).
For bryozoans, with the exception of Scrupocellaria curacaoensis, which represents the first record for the country and was originally described for Curaçao (Fransen 1986), the remaining species found in this study had all been previously cited for Brazil (Vieira et al. 2008). Excepting for Beania kuglei and Nolella stipata, which were already recorded for Pernambuco, all remaining species are being recorded for the first time for this state. Three species had the second record for the country: Buskia socialis, Caulibugula hastingsae, and Scrupocellaria maderensis. The first two were previously reported for the Southeast region (Vieira et al. 2008) and S. maderensis had only been recorded once in Brazil, at Saint Peter and Saint Paul Archipelago (Busk 1884, Edwards & Lubbock 1983). It is possible that their distribution along the Brazilian shelf is broader, yet the absence of other citations might be due to the small amount of studies for this taxonomic group and for the depths where they usually are found.
The most conspicuous bryozoan present on the hard surfaces of the shipwreck, Steginoporella magnilabris, is reported mainly in shallow waters, on piles, shells, sponges, and corals, up to 25 m deep (Osburn 1914). It was also found on experimental rubber and concrete modules, which were immersed up to 9 m deep in Rio de Janeiro State (Zalmon & Gomes 2003) and reported for two shipwrecks in Pernambuco State (Amaral et al. 2010) The habit observed for the bryozoan Nolella stipata, previously cited for Pernambuco by Marcus (1955) and Farrapeira et al. (2009), is commonly reported elsewhere. It has a cryptic habit and creeps over shells, algae, and the stems of hydroids and other bryozoans (Winston 1982), and was already found on shipwrecks (Wendt et al. 1989).
Among the echinoderms found, all of them cited for Pernambuco by Fernandes et al. (2002), the species Linckia guildingii and Oreaster reticulatus deserve to be highlighted as they might become extinct in the near future (MMA... 2004). The occurrence of both species in the wreck demonstrates the importance of these artificial environments, which create niches for their development. It also signals the need for preserving these areas in order to facilitate the restoration of their stocks in the natural environment.
Finally, on the ascidians species, Aplidium lobatum and Microcosmus exasperatus had already been cited for Pernambuco by Millar (1977) and had recently been mentioned on two shipwrecks of Pernambuco coast (Amaral et al. 2010). Microcosmus exasperatus lives on gravel (Millar 1977), on mangrove roots, on coral reefs (Collin et al. 2005), in aggregations or clusters with other ascidians, or attached to various other invertebrates on wharves and bridges (Cole & Vorontsova 1998). This species seems to be well adapted to live on artificial substrata; it has been mentioned on drill ship hulls (Ferreira et al. 2006) and on experimentally placed plates in harbor areas (Marins et al. 2010), both in Rio de Janeiro State, as well as on a shipwreck, in Australia (Walker et al. 2007).
Regarding the distribution of the organisms found we believe one of the decisive factors is the different gradation of light and water speed. The corals were predominant in the areas most sheltered from the currents and with greater light exposure; i.e., the external surface on the western side of the hull and the internal surface of the eastern side. Glasby & Connell (2001) summarized several studies which showed species strongly affected by substrate orientation, levels of light exposure, sedimentation rate, and degree of protection from physical and chemical disturbances. Bachtiar (2000) stated that one of the variables that can interfere in the number of species is the rate of sediment accumulation, which can influence recruitment and development. Moreover, for epifauna there is a significant difference between environments with and without light, as observed by Walker et al. (2007) for an Australian shipwreck.
The Pirapama shipwreck can be compared to an island because it contributes to the success of the settlement of larvae that were previously being lost due to the scarcity of appropriate substrates. The situation found in the shipwreck studied, settled on a sandy bottom, is similar to what was observed by Dokken et al. (2000) when researching the fouling communities of oil platforms from the Gulf of Mexico; these are located in areas of fine sediment where natural hard substrates are not abundant topographical features. Those authors believe that offshore oil/gas production structures provide "islands of opportunity" for organisms that require hard surfaces on which to settle, and eventually create dynamic artificial reefs that meet the habitat needs of mobile reef organisms - both invertebrate and vertebrate. Any new substrate with characteristics favorable to larval settlement in adequate environments is rapidly occupied by epibenthic communities that increment the local trophic chain and promote the development of higher trophic levels (Bruno & Bertness 2001, Witman & Dayton 2001).
Artificial reefs can become genuine biodiversity reservoirs and help conserve species threatened by anthropogenic interferences. Conversely, Work et al. (2008) observed that artificial substrates favored the emergence of invasive species that eliminated native reef species - similar to what happened in Rio de Janeiro with the introduction of Tubastraea coccinea Lesson, 1829 and T. tagusensis Wells, 1982 and their negative interference on natural coral and octocoral communities (DePaula & Creed 2005, Creed 2006) - and suggested that these structures should be removed from natural environments. Thus, it is extremely important to continuously carry out qualitative and quantitative biological monitoring of artificial reefs, for which the ecological role in the maintenance of natural coastal reefs is still unknown.
Acknowledgments
We thank the CNPq for providing a two-year scholarship for the first author and a research scholarship to the third author; Joel Calado and Projeto Mar for sponsorship and infrastructure; Jonata Arruda for identifying the mollucs; Eduardo Esteves, Josivete Pinheiro, and Cecília Pascelli for aid in sponge identification; Tito Lotufo and Gledson Fabiano Ferreira for identifying the ascidians; Vanessa Almeida and Leandro Vieira for identifying the bryozoans and Alvaro Migotto and Danilo Oliveira for helping with hydroid identification. Special thanks are due to Andrea Steiner for her help with the English version and to the anonymous reviewers, who provided valuable comments on the manuscript.
Received 05/05/2010
Revised 14/10/2010
Accepted 24/11/2010
- ABBOTT, R.T. 1974. American seashells. 2nd ed. Van Nostrand Reinhold Co., New York.
- ALMEIDA, L.G. 2007. Levantamento taxonômico dos organismos macrobentônicos incrustantes em um recife artificial marinho, Guarapari - ES. In: Proceedings of the 12th Congresso Latino-Americano de Ciências do Mar, Florianópolis, abril de 2007. Florianópolis: Associação Brasileira de Oceanografia, p. 1-4.
- AMARAL, F.D., FARRAPEIRA, C.M.R. LIRA, S.M. & RAMOS, C.A. 2010. Benthic macrofauna inventory of two shipwrecks from Pernambuco coast, Northeastern of Brazil. Rev. Nordest. Zool. 4(1):24-41.
- AMARAL, F.M.D., RAMOS, C.A.C., LEÃO, Z.M.A.N., KIKUCHI, R.K.P., LIMA, K.K.M., LONGO, L.L., CORDEIRO, R.T.S., LIRA, S.M.A. & VASCONCELOS, S.L. 2009a. Checklist and morphometry of benthic cnidarians from the Fernando de Noronha Archipelago, Brazil. Cah. Biol. Mar. 50:277-290.
- AMARAL, F.M.D., ROCHA, C., FARRAPEIRA, C.M.R., ALVES, M.S., PINTO, S.L., LIRA, S.M.A., LIMA, K.K.M., RAMOS, C.A.C., SANTOS, E.C.L., MOURA, J.R., OLIVEIRA, D.A.S., VERÇOSA, M.M., MELO, A.V.O.M., OLIVEIRA, A.P.A., GONÇALVES, E.F. 2009b. Distribuição espacial de invertebrados bentônicos infralitorais. In O Arquipélago de São Pedro e São Paulo: 10 anos de Estação Científica. (D. L. Viana, F.H.V. Hazin & M.A.C. Souza, Org.). SECIRM, Brasília, p. 148-156.
- AMARAL, F.D., SILVEIRA, S.E.M., VASCONCELOS, S.L. & RAMOS, C.A.C. 2006. Biodiversidade de cnidários bentônicos. In Arquipélago de São Pedro e São Paulo: histórico e recursos naturais (T. Vaske Jr., R.P. Lessa, M.F. Nóbrega, F.M.D. Amaral & S.E.M. Silveira, eds.). Livro Rápido, Olinda, p. 42-55.
- AZEVEDO, F.B.B., CARLONI, G.G. & CARVALHEIRA, L.V. 2006. Colonization of benthic organisms on different artificial substratum in Ilha Grande bay, Rio de Janeiro, Brazil. Braz. Arch. Biol. Technol. 49(2):263-275. doi: 10.1590/S1516-89132006000300012.
- BACHTIAR, I. 2000. Promoting recruitment of scleractinian corals using artificial substrate in the Gilli Indah, Lombok Barat, Indonesia. In: Proc. 9th Int. Coral Reef Symp Bali, 2000. Bali: International Society for Reef Studies, p. 425-430.
- BARROSO, R. & PAIVA, P.C. 2007. Amphinomidae (Annelida: Polychaeta) from Rocas Atoll, Northeastern Brazil. Arq. Mus. Nac. 65(3):357-362.
- BAYER, F.M. 1961. The shallow-water Octocorallia of the West Indian region: a manual for marine biologists. Stud. Fauna Curaçao Carib. Is. 12:1-373.
- BAYNES, T.W. & SZMANT, A.M. 1989. Effect of current on the sessile benthic community structure of an artificial reef. Bull. Mar. Sci. 44(2):545-566.
- BIELER, R., MIKKELSEN, P.M., LEE, T. & Ó FOIGHIL, D. 2004. Discovery of the Indo-Pacific oyster Hyotissa hyotis (Linnaeus, 1758) in the Florida Keys (Bivalvia: Gryphaeidae). Molluscan Res. 24:149-159.
- BORTONE, S.A., SAMOILYS, M.A. & FRANCOUR, P. 2000. Fish and macroinvertebrate evaluation methods. In: Artificial reef evaluation; with application to natural marine habitats (W. Seaman Jr., ed.). CRC Press, Boca Raton.
- BROTTO, D.S., KROHLING, W & ZALMON, L.R. 2006. Fish community modeling agents on an artificial reef on the northern coast of Rio de Janeiro - Brazil. Braz. J. Oceanogr. 54(4):205-212. doi: 10.1590/S1679-87592006000300004.
- BRUNO, J.F. & BERTNESS, M.D. 2001. Habitat modification and facilitation in benthic marine communities. In Marine community ecology (M.D. Bertness, S.D. Gaines & M.E. Hay, eds.). Sinauer Associates, Sunderland.
- BULL, A.S. & KENDALL, J.J. JR. 1994. An indication of the process: offshore platforms as artificial reefs in the Gulf of Mexico. Bull. Mar. Sci. 55(2-3):1086-1098.
- BUSK, G. 1884. Report on the Polyzoa collected by H.M.S. Challenger during the years 1873-1876. Part I. The Cheilostomata. Rept. Challenger Exped. Zool. 10(30):1-216.
- CALCINAI, B., BAVESTRELLO, G. AND CERRANO, C. 2004. Dispersal and association of two invasive species in the Indonesian coral reefs: the octocoral Carijoa riisei and the demosponge Desmapsamma anchorata J. Mar. Biol. Ass. U.K. 84(5):937-941. doi: 10.1017/S0025315404010227h.
- CARVALHO, M. 1997. 4 Naufrágios. Rev. Mergulho 2(11):1-2.
- CARVALHO, M. 2010. Naufrágios do Brasil. Disponível em: <http://www.naufragiosdobrasil.com.br>. [01 de maio de 2010]
- CERRANO, C., CALCINAI, B., PINCA S. & BAVESTRELLO, G. 2006. Reef sponges as hosts of biodiversity: cases from North Sulawesi. In: Proc. 10th Int. Coral Reef Symposium, Okinawa, 2006. International Society for Reef Studies, Okinawa, p. 405-427.
- COLE, L. & VORONTSOVA, M. 1998. Species of Pyuridae (Ascidiacea) from South Vietnam. Bull. Mar. Sci. 62(1):1-6.
- COLLIN, R., DIAZ, M.C., NORENBURG, J.L., ROCHA, R.M., SANCHEZ, J.A., SCHULZ, A., SCHWARTZ, M.L. & VALDÉS, A. 2005. Photographic identification guide to some common marine invertebrates of Bocas Del Toro, Panama. Caribb. J. Sci. 41(3): 638-707.
- CONCEIÇÃO, R.N.L., FRANKLIN-JUNIOR, W. & BRAGA, M.S.C. 1997. Recifes artificiais: um incremento na produtividade em comunidades costeiras do Estado do Ceará. In: Proceedings of the Seminário Internacional sobre Pesca Artesanal, Fortaleza, 1997. Universidade Federal do Ceará, Fortaleza, p. 99-111.
- COSTA, M.B.S.F., MALLMANN, D.L.B. & GUERRA, N.C. 2010. Caracterização sedimentológica da área de fundeio de dois naufrágios na Plataforma Continental Pernambucana. Rev. Gest. Cost. Integ. 10(1):49-64.
- CREED, J.C. 2006. Two invasive alien azooxanthellate corals, Tubastraea coccinea and Tubastraea tagusensis, dominate the native zooxanthellate Mussismilia hispida in Brazil. Coral Reefs 25:350.
- CUMMINGS, S. L. 1994. Colonization of a nearshore artificial reef at Boca Raton (Palm Beach County) Florida. Bull. Mar. Sci. (55):1193-1215.
- DEFELICE, R.C., ELDREDGE, L.G. & CARLTON, J.T. 2001. Nonindigenous invertebrates. In A guidebook of introduced marine species in Hawaii (L.G. Eldredge & C.M. Smith, eds.) Bishop Mus. Tech. Rep. 21:1-70.
- DePAULA, A.F. & CREED, J.C. 2005. Spatial distribution and abundance of nonindigenous coral genus Tubastraea (Cnidaria, Scleractinia) around Ilha Grande, Brazil. Braz. J. Biol. 65(4):661-673. doi: 10.1590/S1519-69842005000400014.
- DIPPER, F. 1991. Colonisation and natural changes in a newly established artificial reef in Gulf waters. In Estuaries and coast: Spatial and temporal intercomparison (M. Ellot & J. P. Ducrotoy, eds.). Olsen and Olsen press, p. 259-264.
- DOKKEN, Q.R., WITHERS, K., CHILDS S. & RIGGS, T. 2000. Characterization and comparison of platform reef communities off the Texas coast. Texas Parks and Wildlife Department Artificial Reef Program, Houston.
- DOWLING, R.K. & NICHOL, J. 2001. The HMAS Swan artificial dive reef. Ann. Tourism Res. 28:226-229.
- EDWARDS, A. & LUBBOCK, R. 1983. The ecology of Saint Paul's Rocks (Equatorial Atlantic). J. Zool. 200:51-69.
- ESTON, V.R. MIGOTTO, A.E., OLIVEIRA FILHO, E.C., RODRIGUES, S.A. & FREITAS, J.C. 1986. Vertical distribution of benthic marine organisms on rocky coasts of the Fernando de Noronha Archipelago (Brazil). Bolm. Inst. Oceanogr. 34:37-53.
- FARRAPEIRA, C.M.R. 2009. Barnacles (Crustacea: Cirripedia) of the estuarine and marine areas of the Port of Recife (Pernambuco-Brazil): a monitoring study for bioinvasion. Cah. Biol. Mar. 50(1):119-129.
- FARRAPEIRA, C.M.R. 2010. Shallow water Cirripedia of the northeastern coast of Brazil: the impact of life history and invasion on biogeography. J. Exp. Mar. Biol. Ecol., 392(1-2):210-219. Doi 10.1016/j.jembe.2010.04.021.
- FARRAPEIRA, C.M.R., RAMOS, C.A.C., BARBOSA, D.F., MELO, A.V.O.M., PINTO, S.L., VERÇOSA, M.M., OLIVEIRA, D.A.S. & FRANCISCO, J.A. 2009. Zonación vertical de la macrofauna de sustratos sólidos del estuario del Río Massangana, Bahía de Suape - Pernambuco, Brasil. Biota Neotrop. 9(1): http://www.biotaneotropica.org.br/v9n1/en/fullpaper?bn01609012009+es (last access in 20/03/2010). doi: 10.1590/S1676-06032009000100011.
- FERNANDES, M.L., TOMMASI, L.R. & LIMA, E.J.B. 2002. Filo Echinodermata de Pernambuco. In Diagnóstico da biodiversidade de Pernambuco (M. Tabarelli & J.M.P. Silva, eds.), vol. 2. Massangana, Recife, p.375-384.
- FERREIRA, C.E.L., GONÇALVES, J.E.A. & COUTINHO, R. 2006. Ship hulls and oil platforms as potential vectors to marine exotic introduction. J. Coast. Res. 39:1340-1345.
- FLOERL O., POOL, T.K. & INGLIS G.J. 2004. Positive interactions between nonindigenous species facilitates transport by human vectors. Ecol. Appl. 14(6):1724-1736. doi: 10.1890/03-5399.
- FRANSEN, C.H.J.M. 1986. Caribbean Bryozoa: Anasca and Ascophora Imperfecta of the inner bays of Curaçao and Bonaire. Stud. Fauna Curaçao Carib. Is. 68(118):1-119.
- GLASBY, T.M. & CONNELL, S.D. 2001. Orientation and position of substrata have large effects on epibiotic assemblages. Mar. Ecol. Prog. Ser. 214:127-135. doi: 10.3354/meps214127.
- HISCOCK, K., SHARROCK, S., HIGHFIELD, J. & SNELLING, D. 2010. Colonization of an artificial reef in south-west England-ex-HMS 'Scylla'. J. Mar. Biol. Ass. U.K. 90(1):69-94. doi: 10.1017/S0025315409991457.
- HIXON, M.A. & BEETS, J.P. 1989. Shelter characteristics and Caribbean fish assemblages: experiments with artificial reefs. Bull. Mar. Sci. 44(2):666-680.
- KELMO, F., ATTRILL, M.J. & JONES, M.B. 2003. Effects of the 1997-1998 El Niño on the cnidarian community of a high turbidity coral reef system (northern Bahia, Brazil). Coral Reefs 22:541-550.
- KNOWLTON, N. 2008. Coral reefs. Curr. Biol. 18(1):18-21.
- KROHLING, W., BROTTO, D.S. & ZALMON, I.R. 2006. Functional role of fouling community on an artificial reef at the northern coast of Rio de Janeiro State, Brazil. Braz. J. Oceanogr. 54(4):183-191. doi: 10.1590/S1679-87592006000300002.
- LABOREL, J.L. 1969. Les peuplements de madreporaires des côtes tropicales du Brésil. Ann. Univ. d'Abidjan - Ser. E- II (3):1-261.
- LEWBEL, G.S., HOWARD, R.L. & GALLAWAY, B.J. 1987. Zonation of dominant fouling organisms on northern Gulf of Mexico petroleum platforms. Mar. Environ. Res. 21(3):199-224. doi: 10.1016/0141-1136(87)90066-3.
- MARCUS, E. 1955. Notas sobre briozoos marinhos brasileiros. Arq. Mus. Nac. 42(1):273-341.
- MARINS, F.O., NOVAES, R.L.M., ROCHA, R.M. & JUNQUEIRA, A. 2010. Non indigenous ascidians in port and natural environments in a tropical Brazilian bay. Zoologia 27(2):213-221. doi: 10.1590/S1984-46702010000200009.
- MASSIN, C.L., NORRO, A. & MALLEFT, J. 2002. Biodiversity of a wreck from the Belgian Continental Shelf: Monitoring using scientific diving. Preliminary results. Bull. Inst. R. Sci. Nat. Belg. Biol. 72:67-72.
- MATTHEWS, H.R. & KEMPF, M. 1970. Moluscos marinhos do Norte e Nordeste do Brasil. II. Moluscos do Arquipélago de Fernando de Noronha (com algumas referências ao Atol das Rocas). Arq. Ciênc. Mar 10(1):1-53.
- MAYAL, E.M., PENNA, O. & RODRIGUES, E. 2002. Hidróides do Estado de Pernambuco. In Diagnóstico da biodiversidade de Pernambuco (M. Tabarelli & J.M.P. Silva, eds.), vol. 2. Massangana, Recife, p.375-384.
- MILLAR, R.H. 1977. Ascidians (Tunicata: Ascidiacea) from the northern and north-eastern Brazilian shelf. J. Nat. Hist. 11(2):169-223. doi: 10.1080/00222937700770131.
- M.M.A. 2004. Instrução normativa Nº 5, de 21 de maio de 2004 - Lista nacional das espécies de invertebrados aquáticos e peixes ameaçadas de extinção. Ministério de Meio Ambiente, Brasília.
- MOTHES, B., LERNER, C. & SILVA, C.M.M. 2003. Guia ilustrado - esponjas marinhas - costa sul-brasileira. USEB, Pelotas.
- MURICY, G. & HADJU, E. 2006. Porifera Brasilis; guia de identificação das esponjas marinhas mais comuns do sudeste do Brasil. Museu Nacional, Rio de Janeiro.
- NELSON, W. G., SAVERCOOL, D.M., NEFF, T., NAVRATIL, P. & RODDA, J. 1994. Disturbance effects on marine infaunal near stabilised oil-ash reefs: spatial and temporal alteration of impacts. Bull. Mar. Sci. 55(2-1):1303-1315.
- NEVES, B.M., LIMA, E.J.B. & PÉREZ, C.D. 2007. Brittle stars (Echinodermata: Ophiuroidea) associated with the octocoral Carijoa riisei (Cnidaria: Anthozoa) from the littoral of Pernambuco, Brazil. J. Mar. Biol. Ass. U.K. 87(5):307-312. doi: 10.1017/S0025315407056263.
- NYBAKKEN, J.W. 1993. Marine biology: an ecological approach. 3rd ed. Harper Collins, New York.
- OSBURN, R.C. 1914. Bryozoa of the Tortuga Island, Florida. Carnegie Inst. Washington Publ., 5(182):181-222.
- PÉREZ, C.D. 2002. Octocorais (Cnidaria, Octocorallia) do litoral pernambucano (Brasil). In Diagnóstico da biodiversidade de Pernambuco (M. Tabarelli & J.M.P. Silva, eds.), vol. 2. Massangana, Recife, p.365-368.
- PERKOL-FINKEL, S. & BENAYAHU, Y. 2004. Community structure of stony and soft corals on vertical unplanned artificial reefs in Eilat (Red Sea): comparison to natural reefs. Coral Reefs, 23: 195-205. doi: 10.1007/s00338-004-0384-z.
- PICKERING, H. & WHITMARSH, D. 1997. Artificial reefs and fisheries exploitation: a review of the 'attraction versus production' debate, the influence of design and its significance for policy. Fish. Res. 31:39-59.
- PICKERING, H., WHITMARSH, D. & JENSEN, A. 1998. Artificial reefs as a tool to aid rehabilitation of coastal ecosystems: Investigating the potential. Mar. Pollut. Bull. 37:505-514. doi: 10.1016/S0025-326X(98)00121-0.
- PONTI, M., ABBIATI, M. & CECCHERELLI, V.U. 2002. Drilling platforms as artificial reefs: Distribution of macrobenthic assemblages of the ''Paguro'' wreck (northern Adriatic Sea). Icea J. Mar. Sci. 59(Suppl.):S316-S323. doi:10.1006/jmsc.2002.1225.
- RIOS, E.C. 2009. Compendium of Brazilian sea shells. Evangraf, Porto Alegre.
- ROSS, A. 1969. Studies on the Tetraclitidae (Cirripedia: Thoracica). Revision of Tetraclita Trans. San Diego Soc. Nat. Hist., 15(15):237-251.
- RUSSELL, B.C. 1975. The development and dynamics of a small artificial reef community. Helgol. wiss. Meeresunters. 27(3):298-312. doi: 10.1007/BF01611698.
- SANTOS, D.H.C & PASSAVANTE, J.Z.O. 2007. Recifes artificiais marinhos: modelos e utilizações no Brasil e no Mundo. Bol. Tec. Cient. CEPENE 15(1):113-124.
- SANTOS, D.H.C., HAZIN, F.V., FISHER, A.F., FEITOSA, F.N. & ARAÚJO, M.E. 2008. The creation of a shipwreck park off the coast of Pernambuco, Brazil. Repesca. 3(1):90-97.
- SEAMAN, W. (ED) 2000. Artificial reef evaluation with application to natural marine habitats. CRC Press, Boca Raton.
- SCHEFFER, A. 2001. Estrutura e dinâmica de comunidades epilíticas de habitats artificiais e suas relações com os fatores ambientais na plataforma rasa do Estado do Paraná. Dissertation, Universidade Federal do Paraná, Curitiba, Brazil.
- SILVA, B.T. & PÉREZ, C.D. 2002. Diagnosis del conocimiento de la fauna de octocorales (Cnidaria, Anthozoa) de la región Nordeste de Brasil. Trop. Oceanogr. 30:15-22.
- SNELGROVE, P.V.R. 1998. The biodiversity of macrofaunal organisms in marine sediments. Biodivers. Conserv. 7:1123-1132.
- SOUZA, J.R.B., RODRIGUES, H.A., NEVES, B.M. & PEREZ, C.D. 2007. First report of bristleworm predator of the reef octocoral Carijoa riisei Coral Reefs 26(4):1033. doi: 10.1007/s00338-007-0290-2.
- TENÓRIO, D.O., LUZ, B.R.A. & MELO, W.R. 2000. Moluscos marinhos do litoral do Estado de Pernambuco. In Diagnóstico da biodiversidade de Pernambuco (M. Tabarelli & J.M.P. Silva, eds.), vol. 2. Massangana, Recife, p.493-528.
- VIEIRA, L.M., MIGOTTO, A.E. & WINSTON, J.E. 2008. Synopsis and annotated checklist of recent marine Bryozoa from Brazil. Zootaxa 1810:1-39.
- WAGNER, D., KAHNG, S.E. & TOONEN, R.J. 2009. Observations on the life history and feeding ecology of a specialized nudibranch predator (Phyllodesmium poindimiei), with implications for biocontrol of an invasive octocoral (Carijoa riisei) in Hawaii. J. Exp. Mar. Biol. Ecol. 372(1-2):64-74. doi: 10.1016/j.jembe.2009.02.007.
- WALKER, S.J., SCHLACHER, T.A. & SCHLACHER-HOENLINGER, M.A. 2007. Spatial heterogeneity of epibenthos on artificial reefs: fouling communities in the early stages of colonization on an East Australian shipwreck. Mar. Ecol. 28(4):435-445. doi:10.1111/j.1439-0485.2007.00193.x.
- WENDT, P.H., KNOTT, D.M. & VAN DOLAH, R.F. 1989. Community structure of the sessile biota on five artificial reefs of different ages. Bull. Mar. Sci. 44(3):1106-1122.
- WHOI 1952. Marine fouling and its prevention. Woods Hole Oceanographic Institution - WHOI, Annapolis.
- WIGGERS, F. & MAGALHÃES, A.R.M. 2003. Novas ocorrências de moluscos no litoral de Santa Catarina, Brasil. Biotemas 16(1):81-89.
- WINSTON, J.E. 1982. Marine bryozoans (Ectoprocta) of the Indian River area, Florida. Bull. Am. Mus. Nat. Hist. 173:99-176.
- WIRTZ, P., ARAÚJO, R. & SOUTHWARD, A.J. 2006. Cirripedia of Madeira. Helgol. Mar. Res. 60(3):207-212. doi: 10.1007/s10152-006-0036-5.
- WITMAN, J.D. & DAYTON, P.K. 2001. Rocky subtidal communities. In Marine community ecology (M.D. Bertness, S.D. Gaines & M.E. Hay, eds.). Sunderland, Massachusetts.
- WOODHEAD, P.M.J. & JACOBSON, M.E. 1985. Epifaunal settlement, the processes of community development and succession over two years on an artificial reef in the New York Bight. Bull. Mar. Sci. 37(1):364-376.
- WORK, T.M., AEBY, G.S. & MARAGOS, J.E. 2008. Phase shift from a coral to a corallimorph-dominated reef associated with a shipwreck on Palmyra Atoll. PLoS One 3(8):e2989. doi: 10.1371/journal.pone.0002989.
- YAN, T., YAN, W.-X., DONG, Y., WANG, H.-J., YAN, Y. & LIANG, G.-H. 2006. Marine fouling of offshore installations in the northern Beibu Gulf of China. Int. Biodeterior. Biodegrad. 58(2):99-105.
- YOUNG, P.S. 1998. Maxillopoda. Thecostraca. In Catalogue of Crustacea of Brazil (P.S. Young, ed.). Museu Nacional do Rio de Janeiro, Rio de Janeiro, p.263-285.
- ZALMON, I.R. & GOMES F.A. 2003. Comunidade incrustante em diferentes materiais de um recife artificial no litoral norte do Estado do Rio de Janeiro. Biotemas 16(1):57-80.
- ZINTZEN, V., MASSIN, C.L., NORRO, A. & MALLEFET, J. 2006. Epifaunal inventory of two shipwrecks from the Belgian Continental Shelf. Hydrobiologia 555(1):207-219. doi: 10.1007/s10750-005-1117-1.
- ZINTZEN, V., NORRO, A., MASSIN, C. & MALLEFET, J. 2008. Spatial variability of epifaunal communities from artificial habitat: Shipwrecks in the Southern Bight of the North Sea. Estuar. Coast. Shelf Sci. 76(2):327-344. doi: 10.1007/s00227-007-0819-5.
Publication Dates
-
Publication in this collection
29 July 2011 -
Date of issue
Dec 2010
History
-
Reviewed
14 Oct 2010 -
Received
05 May 2010 -
Accepted
24 Nov 2010